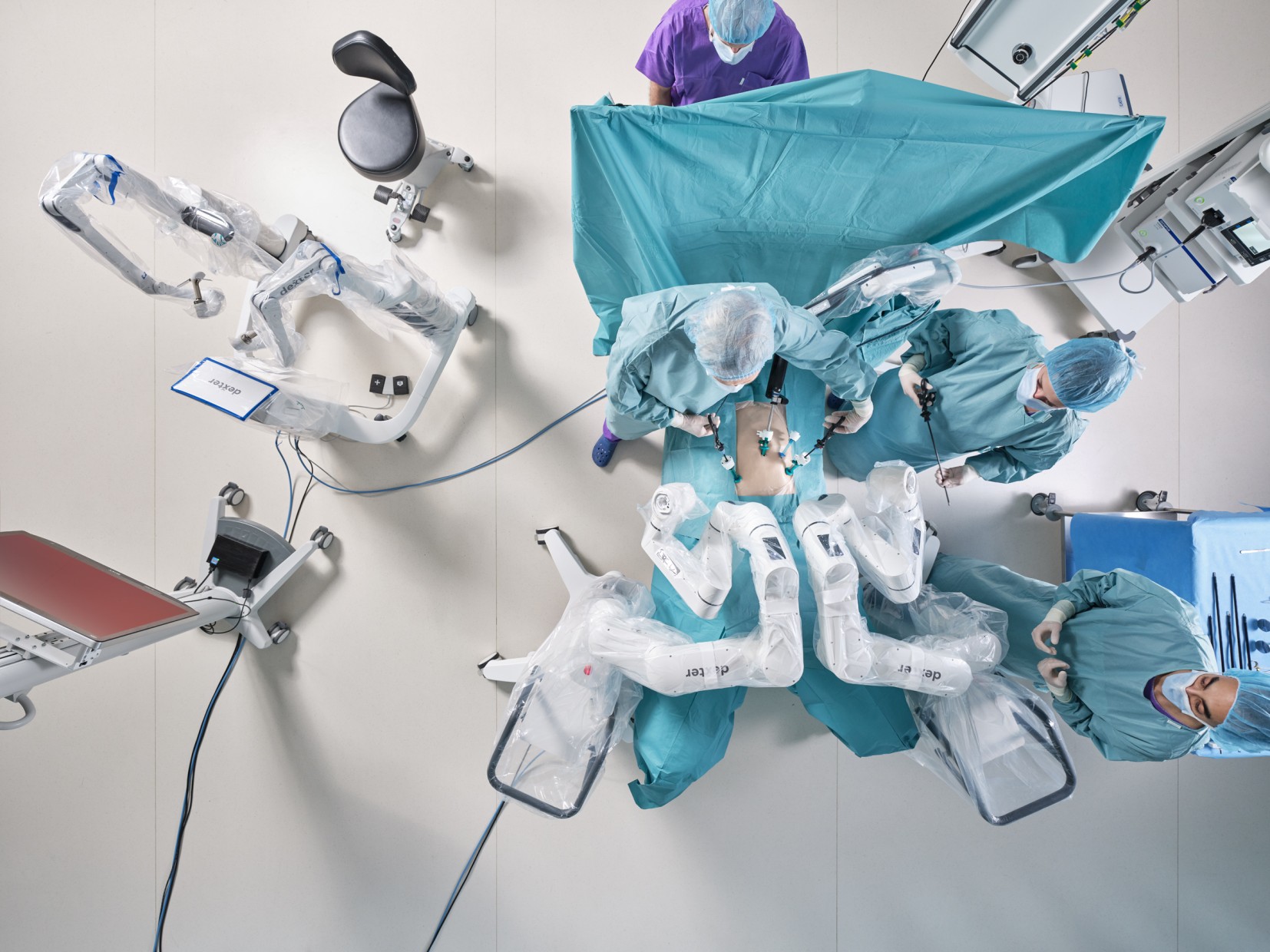
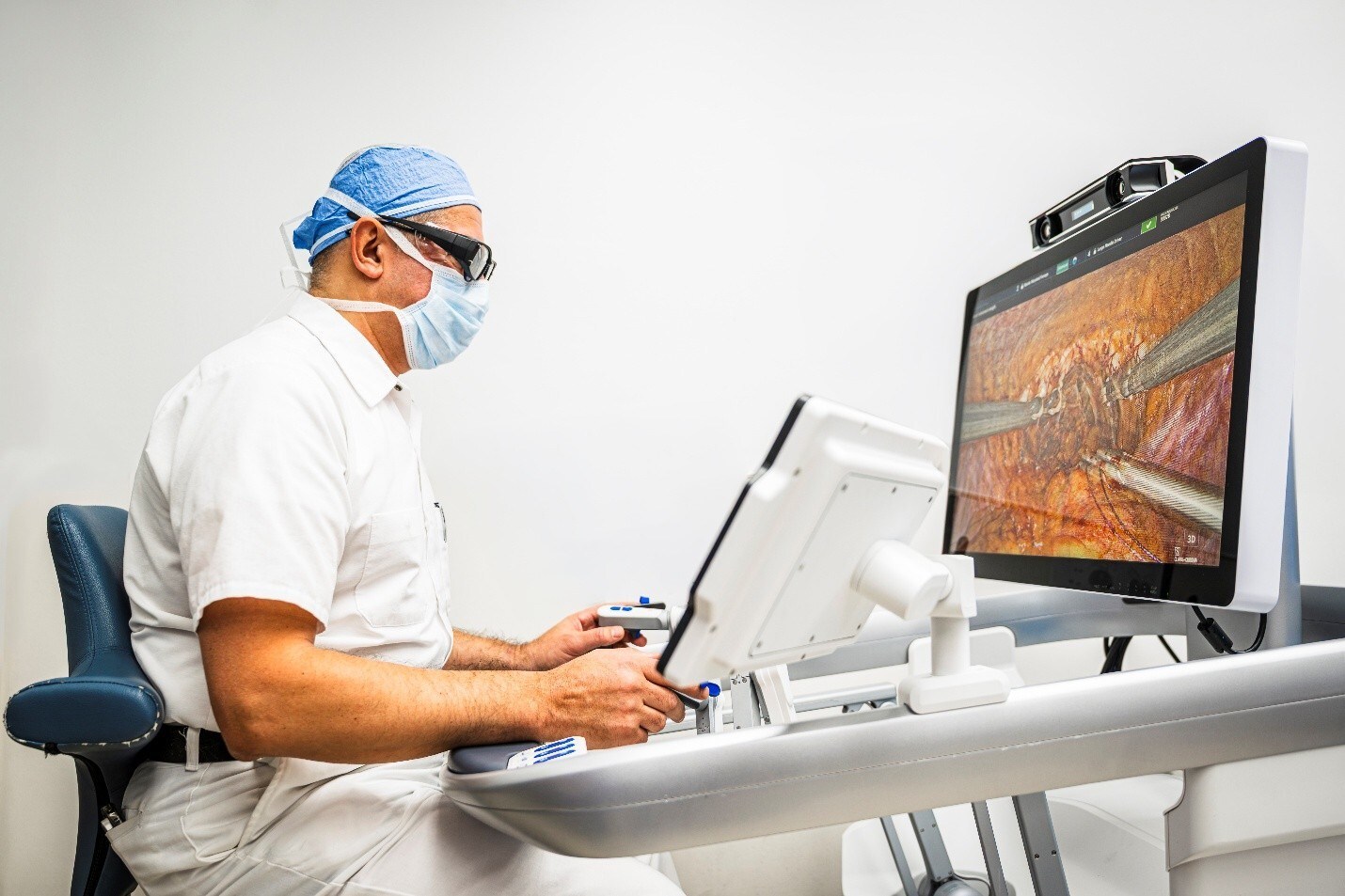
Distalmotion’s DEXTER® Robotic Surgery System Gains FDA Clearance for Hysterectomy and Gynecologic Procedures
FDA 510(k) clearance expands DEXTER’s U.S. indications to include total benign hysterectomy, oophorectomy, salpingectomy, and more—strengthening access to robotic surgery in outpatient sites of care.
Image Courtesy: Public Domain
Distalmotion, the global MedTech company empowering access to the benefits of robotic surgery in outpatient sites of care, announced that it has received FDA 510(k) clearance for the use of its DEXTER® Robotic Surgery System in total benign hysterectomy as well as oophorectomy, salpingectomy, and other gynecologic procedures. This milestone marks the company’s third approved indication in the United States, following prior FDA clearances for inguinal hernia repair and cholecystectomy.
“Hysterectomy is a foundational procedure in women’s health, with over 600,0001 cases performed annually in the US,” said Greg Roche, CEO of Distalmotion. “With FDA clearance for three of the top five soft tissue procedures performed in ambulatory surgery centers2—inguinal hernia, cholecystectomy, and now hysterectomy—DEXTER offers ambulatory surgery centers a right-sized solution to provide the benefits of robotic surgery in a convenient surgical setting.”
As one of the highest volume gynecologic surgeries, hysterectomy is increasingly performed in outpatient sites of care. As more soft tissue procedures migrate to outpatient settings, there is growing demand for robotic solutions that deliver enhanced dexterity, precision, and surgeon ergonomics —without requiring dedicated robotic OR infrastructure or compromising on efficiency and cost.
“Access to robotics has long been a challenge for gynecologists due to limited block time availability,” said Dr. Erica Stockwell, Gynecology Surgeon, AdventHealth Celebration. “DEXTER solves this with a mobile robot that can be shared by various specialties, giving us the access we've been waiting for. Having immediate bedside access to my patient during a robotic procedure also gives me control of the procedure and helps reduce staffing needs.”
Included in this regulatory clearance is an enhanced version of the DEXTER system. These enhancements are designed to simplify operating room setup, streamline procedural workflows, and optimize instrument performance—further strengthening the value of DEXTER in outpatient settings and bringing the system in the US up to the latest global version.
Building on its growing footprint in the US and Europe, DEXTER has been used in over 2,000 procedures across more than thirty procedure types in general, gynecological, colorectal, and urological surgery—including over 250 hysterectomies in Europe. Distalmotion continues to advance its work in gynecology with its recently completed sacrocolpopexy (SPARO) and initiation of its myomectomy (REAL-M) clinical trials.