EndoQuest Robotics Achieves First Robotic ESD by Gastroenterologist in PARADIGM Trial
Pioneering endoluminal surgical technology enables gastroenterologists to perform complex colorectal procedures with enhanced precision, reproducibility, and reduced learning curves.
Image Courtesy: Public Domain
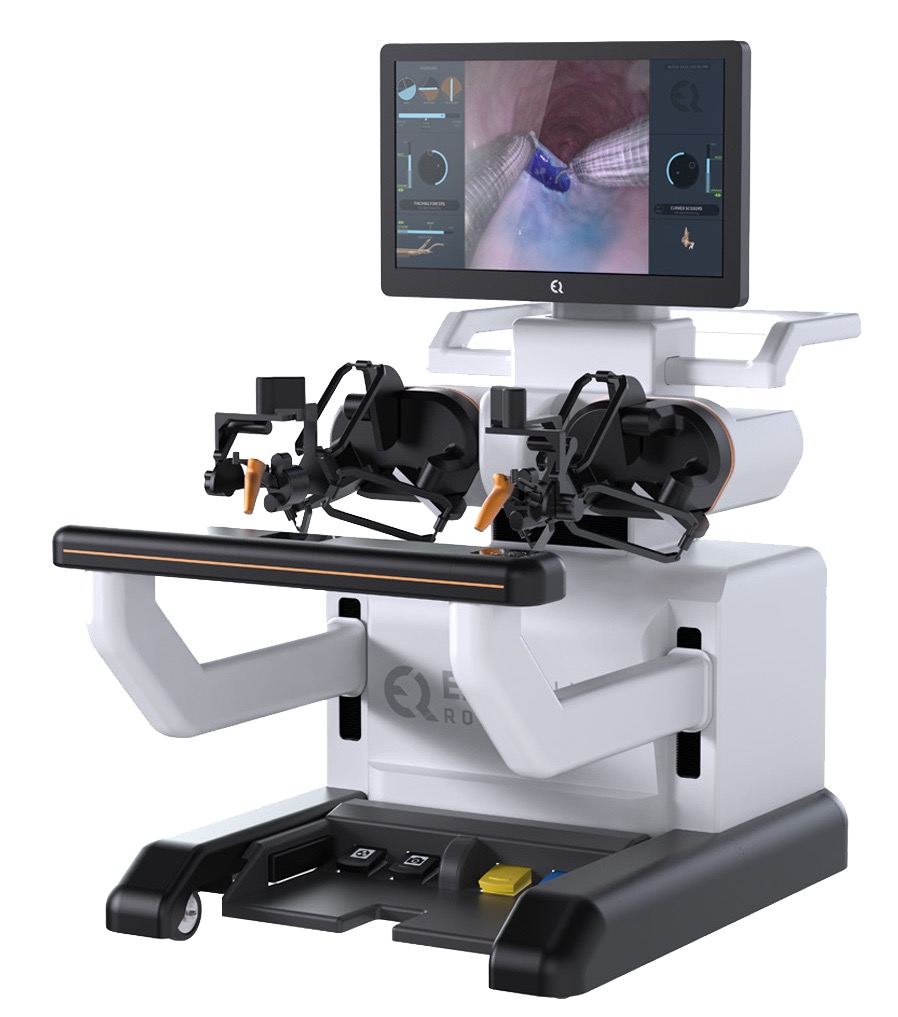
EndoQuest Robotics, Inc., a pioneering leader in endoluminal robotic surgical technology, announced the completion of the first procedure performed by a gastroenterologist in the PARADIGM Trial (Prospective Assessment of a Robotic Assisted Device in Gastrointestinal Medicine). The PARADIGM Trial is a pivotal multicenter study evaluating EndoQuest’s Endoluminal Surgical (ELS) System for use in lower gastrointestinal (GI) tract procedures by both colorectal surgeons and gastroenterologists (Clinicaltrials.gov).
This milestone marks the world’s first fully robotic endoscopic submucosal dissection (ESD) performed by a gastroenterologist in an FDA Investigational Device Exemption (IDE) pivotal trial. EndoQuest recently announced the first PARADIGM Trial cases completed at HCA Houston Healthcare and AdventHealth Orlando, which were performed by colorectal surgeons Dr. Eric Haas and Dr. Matthew Albert, respectively.
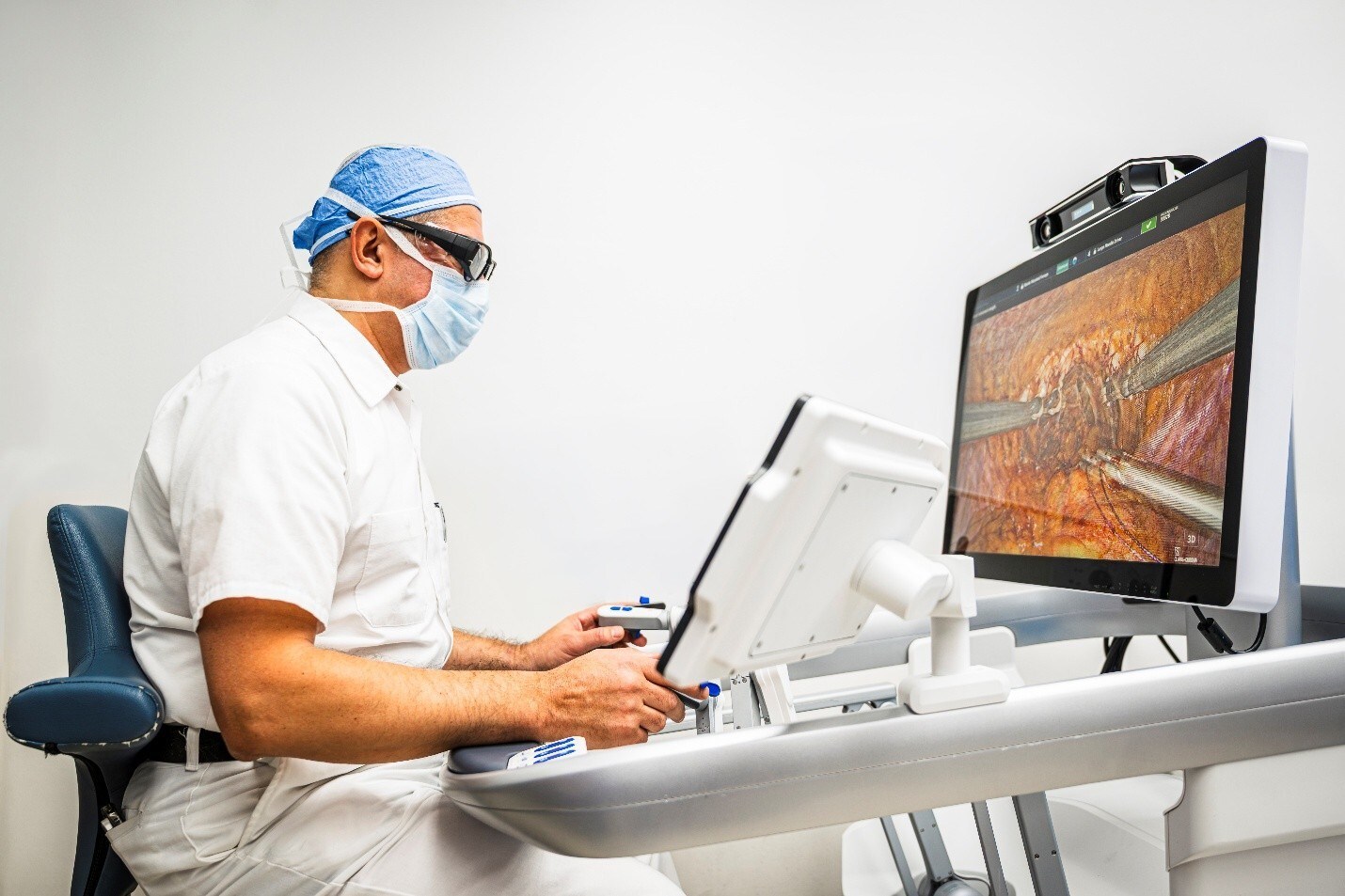
Dr. Norio Fukami, Professor of Medicine and Director of Therapeutic Endoscopy and Interventional Gastrointestinal Endoscopy Fellowship at Mayo Clinic, Arizona, successfully completed a robotic ESD using EndoQuest’s ELS System to remove a complex colorectal lesion measuring 4 cm in size.* Dr. Fukami is a world-renowned expert in ESD and other advanced endoluminal and pancreaticobiliary techniques and was an editor for the first English textbook on ESD.
“The device was instinctive and easy to use, enabling dynamic traction and counter-traction of surgical technique, and made a complex procedure that would traditionally be much more challenging feel relatively straightforward,” said Dr. Fukami. “This technology represents a monumental shift in our ability to perform ESD and other advanced endoluminal therapies. For the first time, we’re equipped with a true surgeon's ‘second hand’ that makes these procedures more natural and reproducible, with the potential to significantly reduce patients' and physicians' burden.”
ESD has emerged as an effective treatment option for early lesions in the GI tract, enabling many patients to avoid invasive surgeries such as colectomies. Despite these clear benefits, ESD and other advanced endoluminal therapies are technically challenging with steep learning curves, due in large part to the inherent limitations of standard flexible endoscopes and instrumentation. The ELS System is designed to make advanced endoluminal procedures easier to learn and perform by enabling surgical tasks and maneuvers not currently possible in advanced therapeutic endoscopy. Pre-clinical data presented by Brigham and Women’s Hospital at Digestive Disease Week (DDW) in 2024 and 2025 demonstrated that the ELS System significantly reduces the learning curve compared to conventional techniques for ESD and full-thickness defect closure among novice gastroenterologists.1, 2
“Therapeutic endoscopy represents one of the fastest-growing areas within medicine,” said Dr. Todd Wilson, Chief Medical Officer and Chairman of the International Advisory Board of EndoQuest Robotics. “With a greater than 50% increase in the number of Advanced Endoscopy Fellowship programs over the past decade, there is clearly a growing interest in advancing endoluminal, organ-sparing care, and our technology is positioned to drive this therapeutic shift.”
“Our passionate team is committed to transforming patient care by empowering a broad spectrum of physicians and procedures with the benefits of robotics,” said Eduardo Fonseca, CEO of EndoQuest Robotics. “EndoQuest is positioned to accelerate adoption of robotics beyond the operating room and into endoscopy suites and ambulatory surgery centers, unlocking significant clinical and commercial value well beyond our initial focus on ESD.”
The PARADIGM Trial is enrolling 50 subjects across five leading U.S. healthcare institutions: Brigham and Women’s Hospital (Boston), Mayo Clinic (Scottsdale), Cleveland Clinic (Cleveland), AdventHealth (Orlando), and HCA Healthcare (Houston). Following trial completion, EndoQuest Robotics plans to submit a De Novo request for authorization to market the ELS System in the U.S.