Microbot Medical’s LIBERTY® Robotic System Showcased at ISET 2026, Highlighting Future of Endovascular Robotics
FDA-cleared single-use robotic system featured in expert Town Hall discussion as demand grows for precision, radiation reduction, and workflow efficiency in vascular procedures.
Microbot Medical Inc. (Nasdaq: MBOT), developer and distributor of the innovative LIBERTY® Endovascular Robotic System, announced that LIBERTY was featured at the International Symposium on Endovascular Therapy (ISET) conference, which took place February 9-12, 2026, in Miami Beach, Florida. The International Symposium on Endovascular Therapy brings together current and emerging endovascular experts to examine new techniques, technologies, and procedures that advance patient care.
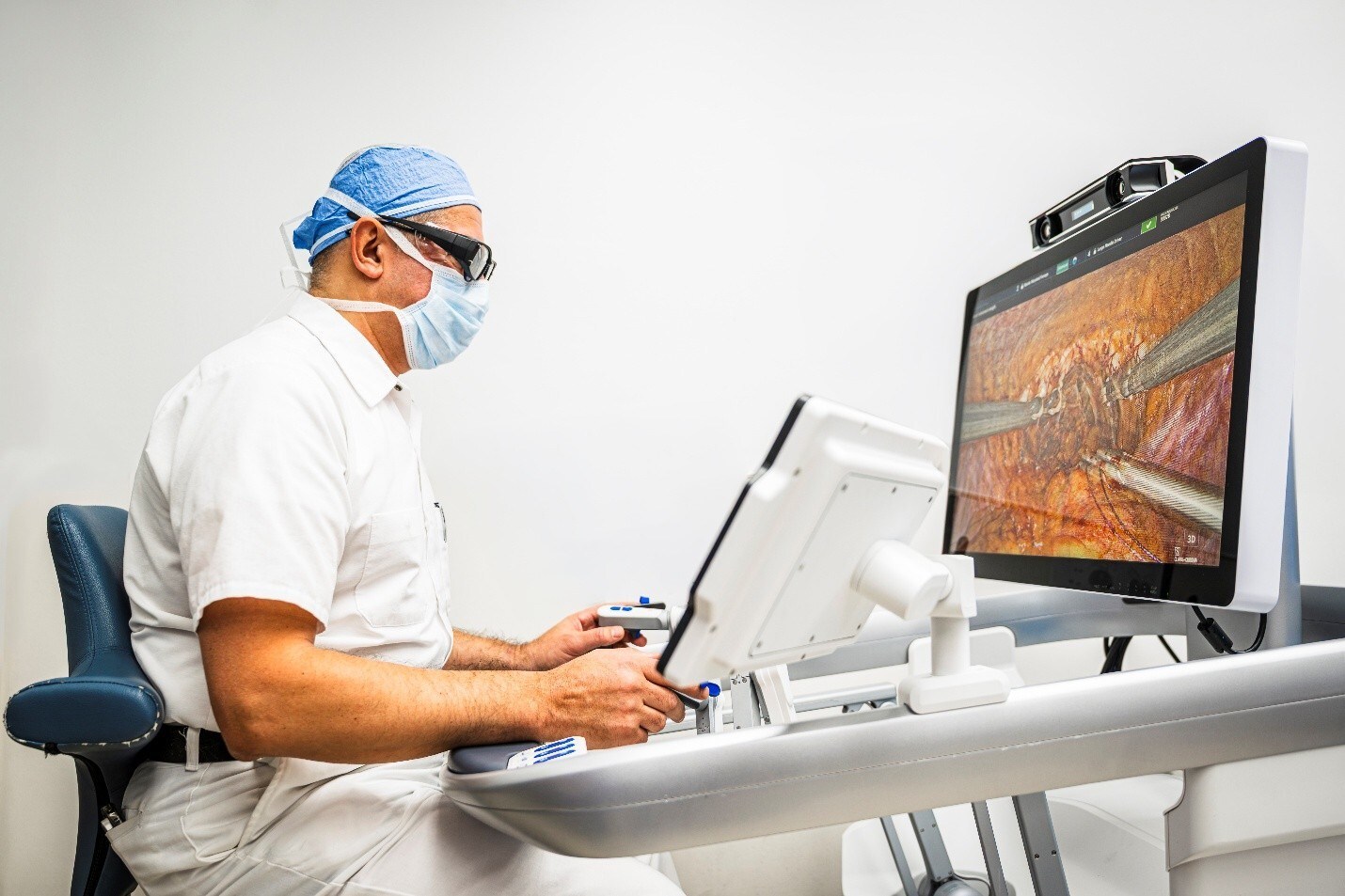
During a Town Hall session, titled Advances in Endovascular Robotics: Will There be a Role in the Future?, which included leading interventional radiologists, interventional cardiologists, and vascular surgeons discussing real-world clinical experience, one of the featured agenda items focused on the future of single-use handheld vascular robots, which specifically highlighted the LIBERTY Endovascular Robotic System. The LIBERTY System, the only FDA cleared, single-use endovascular robotic system, was discussed prominently, highlighting its seamless workflow integration and features, which the Company believes will be key drivers for broader adoption and shape the next phase of peripheral endovascular intervention. As procedures become more complex and operator demands are expected to continue to grow, robotic assistance offers a potential solution to challenges many clinicians face daily.
“ISET plays a meaningful role in shaping the future of endovascular innovation, providing a platform where healthcare leadership and emerging technologies converge. We believe that LIBERTY’s inclusion in this important dialogue confirms the commercial traction of LIBERTY and reinforces our position among a prestigious group of interventional radiologists, vascular surgeons and other clinical decision makers,” commented Harel Gadot, President, CEO & Chairman. “To date, LIBERTY has been used across multiple hospitals for a wide range of procedure types, including complex cases. We have seen increased interest from physicians across several specialties within the same account and network of hospitals, including interventional radiology, interventional oncology, vascular surgery and interventional cardiology, which we believe is reinforcing its broad clinical relevance in the peripheral space and expanding overall commercial opportunity.”
LIBERTY is the only FDA cleared, single-use, remotely operated robotic system for peripheral endovascular procedures, and it is designed for precise vascular navigation while aiming to reduce radiation exposure and physical strain. The Company commenced the limited market release of the LIBERTY system in late 2025 and plans for a full market release at the Society of Interventional Radiology (SIR) conference in April 2026, allowing the Company to showcase LIBERTY with the goal to deepen market adoption.