CMR Surgical’s Versius Plus Robotic System Secures FDA 510(k) Clearance Ahead of U.S. Launch
Regulatory milestone clears path for 2026 commercialization of CMR’s next-generation surgical robot in the United States
Image Courtesy: Public Domain
- Regulatory clearance of CMR’s most advanced system paves the way for upcoming US commercial launch
- Strong clinical interest received from US surgeons nationwide for the system
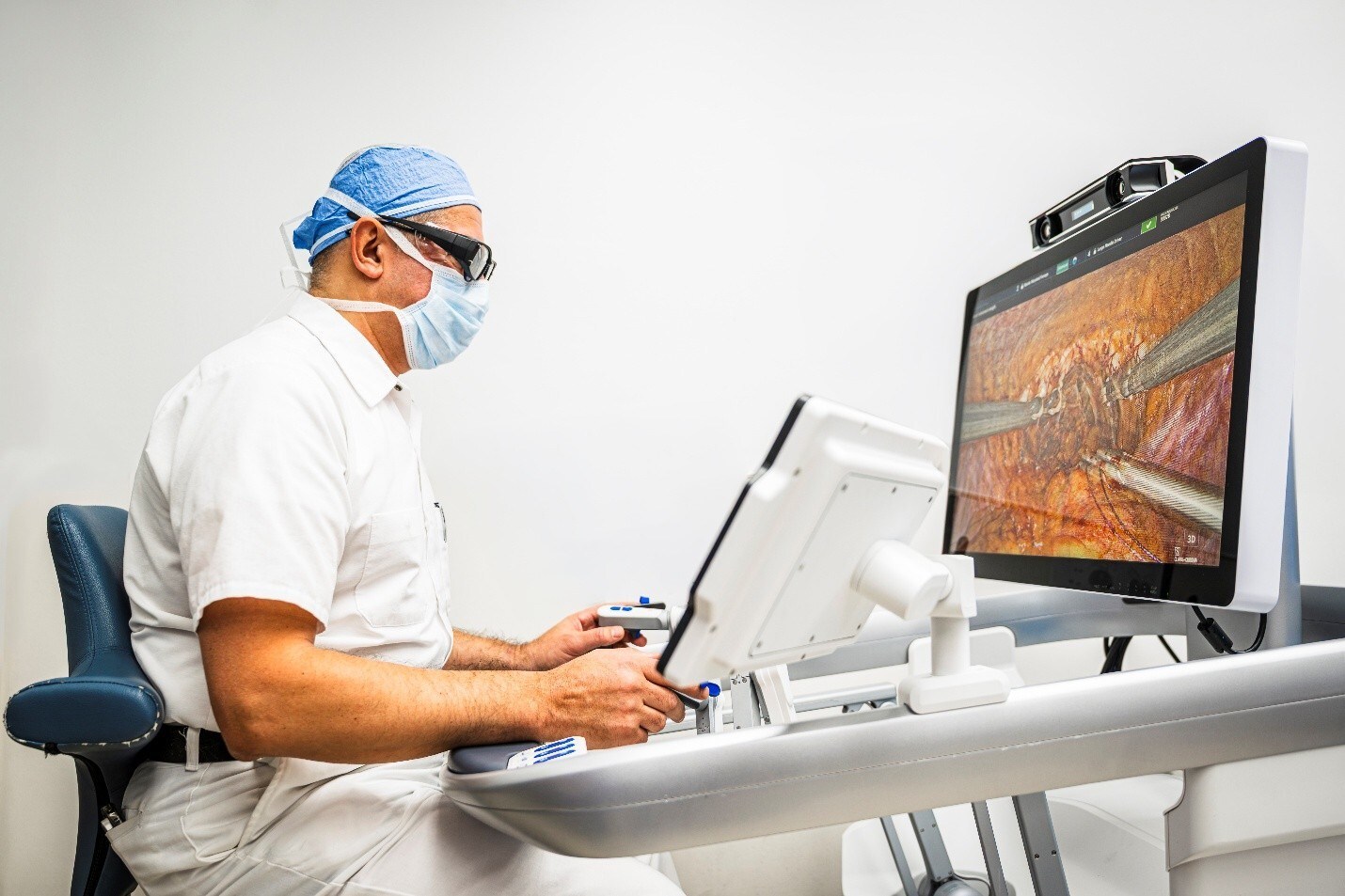
CMR Surgical, a global medical technology company, announces its second-generation surgical robotic platform, Versius Plus, has received 510(k) clearance from the United States Food and Drug Administration (FDA) for cholecystectomy procedures.
With a flexible, modular design and data-driven digital ecosystem, Versius Plus represents a new market offering, providing more choice for surgeons and hospitals. The system brings next-generation innovation to the operating room, designed not only for the surgeon but for the entire OR team. Its open console facilitates communication between the surgeon and other medical professionals, which supports real-time decision making.
The FDA previously granted marketing authorization for CMR’s first-generation Versius Surgical System through the De Novo process in October 2024. With the 510(k) clearance of Versius Plus, CMR is on track to begin commercialization in 2026.
Outside of the United States, CMR’s robotic surgical platforms have already completed over 40,000 surgical procedures, establishing a strong foundation for their performance across multiple specialties and care settings, and making the soft tissue robotic platforms the second most utilized globally.
Massimiliano Colella, Chief Executive Officer at CMR Surgical, commented:
“This 510(k) clearance represents an exciting new chapter for CMR Surgical as we introduce Versius Plus to the U.S. market. Built on years of global clinical use data, Versius Plus delivers the flexibility and intelligence today’s healthcare institutions need to advance robotic-assisted surgery. It’s inspiring to see our new technology transforming the landscape of surgical care.”
Chris O’Hara, President & General Manager US at CMR Surgical, commented:
“Versius Plus is designed to meet the practical realities of today’s healthcare environment — adaptable to different settings, efficient to integrate, and scalable for long-term growth. FDA clearance represents an exciting opportunity to partner with healthcare systems across the U.S. Versius Plus is designed to support a broad range of soft-tissue procedures, and we are diligently advancing additional indications in the U.S. and aim to help make robotic-assisted surgery more accessible than ever before.”
Key Benefits of Versius Plus
Adaptable
Owing to its compact, modular design, Versius Plus moves easily between departments and operating rooms – with no dedicated OR required. Hospitals can seamlessly switch between robotic and non-robotic procedures in the same space. Surgeons benefit from flexible port placement, choosing the setup that best fits their patient and their technique.
Versatile
Through design and technology, Versius Plus delivers the precision and efficiency surgeons’ demand. Its integrated fluorescence visualisation system, vLimeLite, enables real-time ICG imaging, delivering multiple visualization modes with overlay and grayscale options. With a full surgical toolkit, Versius Plus is ready to transform surgical experiences in the OR.
Digitally-Driven
The Versius Plus ecosystem puts data at the user’s fingertips with Versius Connect: A dedicated surgeon app with a near-real-time logbook of procedures and Versius Team: A live dashboard for surgical teams and hospitals, tracking usage, case volume, and system efficiency to help optimize robotics programs.