Medtronic Launches U.S. Clinical Study to Evaluate Hugo™ Robotic-Assisted Surgery System in Gynecology
The Embrace Gynecology IDE study marks a major milestone for Medtronic’s Hugo™ RAS system, assessing safety and effectiveness in robotic-assisted hysterectomy procedures across leading U.S. hospitals.
Image Courtesy: Public Domain
Medtronic plc (NYSE: MDT) announced the start of the Embrace Gynecology investigational device exemption (IDE) U.S. clinical study to evaluate the safety and effectiveness of its Hugo™ robotic-assisted surgery (RAS) system in robotic-assisted gynecological procedures.
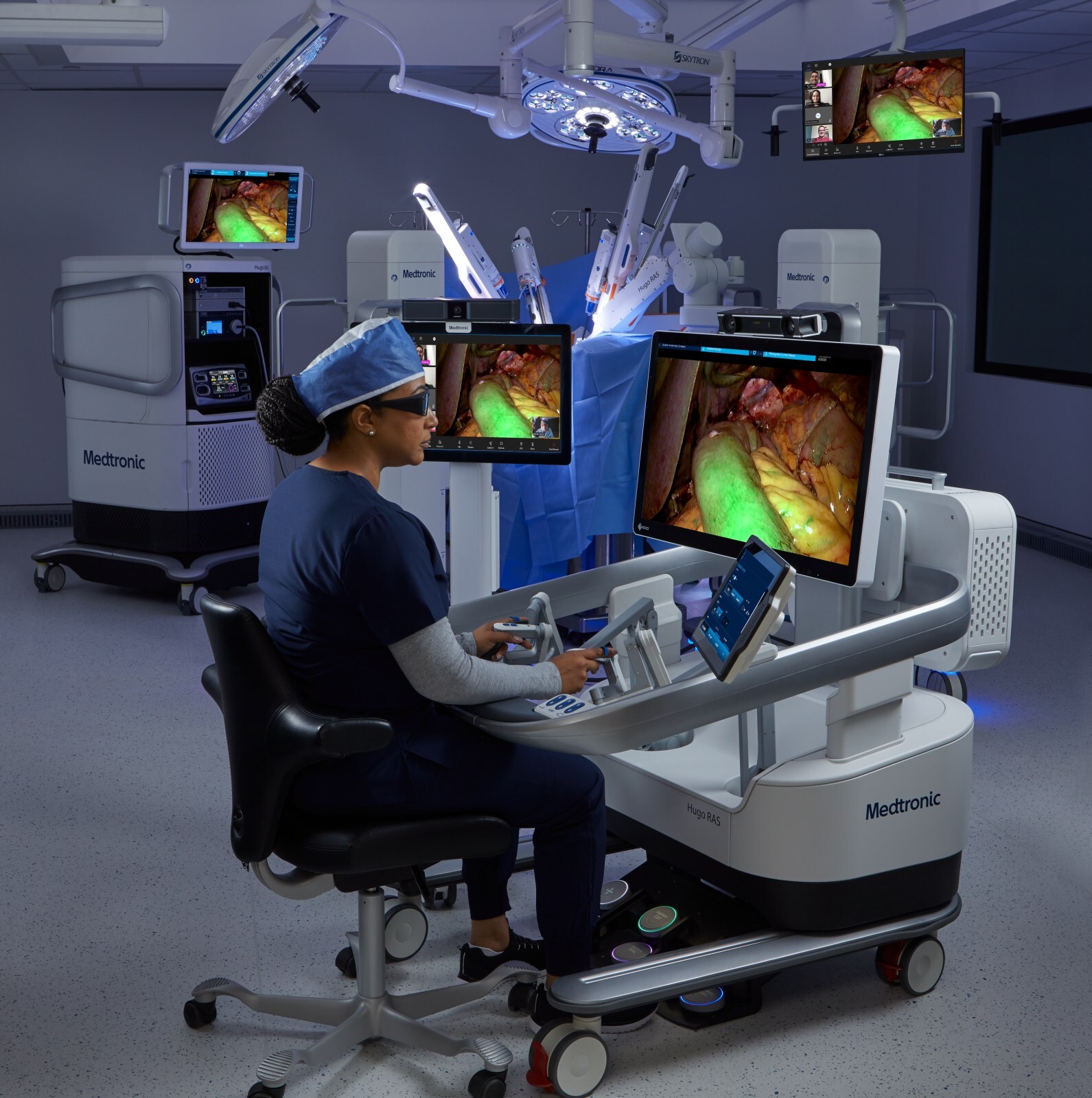
The first procedures, total hysterectomies, were successfully completed at AHN West Penn Hospital in Pittsburgh, PA, by Dr. Sarah Crafton and Dr. Eirwen Miller, Embrace Gynecology study surgeons at Allegheny Health Network.
Embrace Gynecology is a prospective multicenter study to evaluate the safety and effectiveness of the Hugo RAS system when used during hysterectomy procedures (radical, modified radical, or total hysterectomies), inclusive of those being treated for malignancies. The study will enroll up to 70 patients across up to 5 U.S. hospitals.
"We are excited to initiate this important clinical study, which aims to investigate surgical treatment options for women in the U.S.," said Dr. Emma Rossi, Embrace Gynecology national principal investigator and associate professor of Obstetrics and Gynecology at Duke University in Durham, NC. "In my experience, women facing a gynecologic diagnosis want two things: to effectively treat their condition, and to get back to their full lives as quickly as possible. Robotic-assisted surgery helps make that possible."
The National Cancer Institute estimates that nearly 111,000 U.S. women will have been diagnosed with gynecological cancer by the end of 2025, while hundreds of thousands more will experience benign conditions such as fibroids or abnormal bleeding.1 Surgery is a common treatment for many of these gynecological conditions, and for some cancers, such as uterine (endometrial) cancer, it is usually the first and primary treatment.2 As a form of minimally invasive surgery, robotic-assisted procedures offer patients fewer complications, shorter hospital stays, and faster return to normal activities than open surgery.3.4,5
"The study name, Embrace, reflects our deeply felt compassion and care for patients and our commitment to providing access to less invasive treatment options for women," said Dr. James Porter, chief medical officer of Robotic Surgical Technologies and Digital Technologies within the Surgical business of Medtronic. "We are grateful for the strong partnership with clinical teams at our study sites and share their excitement about this rigorous scientific study that is helping to usher in a new era of choice for patients in the U.S."
Embrace Gynecology becomes the third IDE clinical study for Hugo RAS in the U.S., demonstrating Medtronic's commitment to obtaining multiple indications for its Hugo RAS system. The two other U.S. IDE clinical studies — Expand URO and Enable Hernia Repair — both met their primary safety and effectiveness endpoints.
Also, in July, positive results from a Medtronic-sponsored prospective study of the Hugo RAS system in benign gynecologic procedures outside the U.S. were shared at the Society of Robotic Surgery congress in Strasbourg, France.
The company's first U.S. submission, for a urology indication, is under review by the Food and Drug Administration and is expected later in the company's current fiscal year, followed by planned indication expansions into hernia repair and gynecology.
Born out of Medtronic's 75-year commitment to expanding access to minimally invasive surgical options worldwide in partnership with clinicians, the Hugo RAS system is currently in clinical use in more than 30 countries on five continents, with nearly 300 independent publications to date.
The Medtronic Hugo RAS system is commercially available in certain geographies. Regulatory requirements of individual countries and regions will determine approval, clearance, or market availability. In the EU, the Hugo RAS system is CE marked. In the U.S., the Hugo RAS system is an investigational device not for sale.