Distalmotion’s DEXTER® Robotic Surgery System Secures FDA Clearance for Cholecystectomy
Second U.S. Indication Expands Access to Robotic Surgery in Outpatient Settings, Reinforcing DEXTER’s Role in High-Volume General Procedures
Image Courtesy: Public Domain
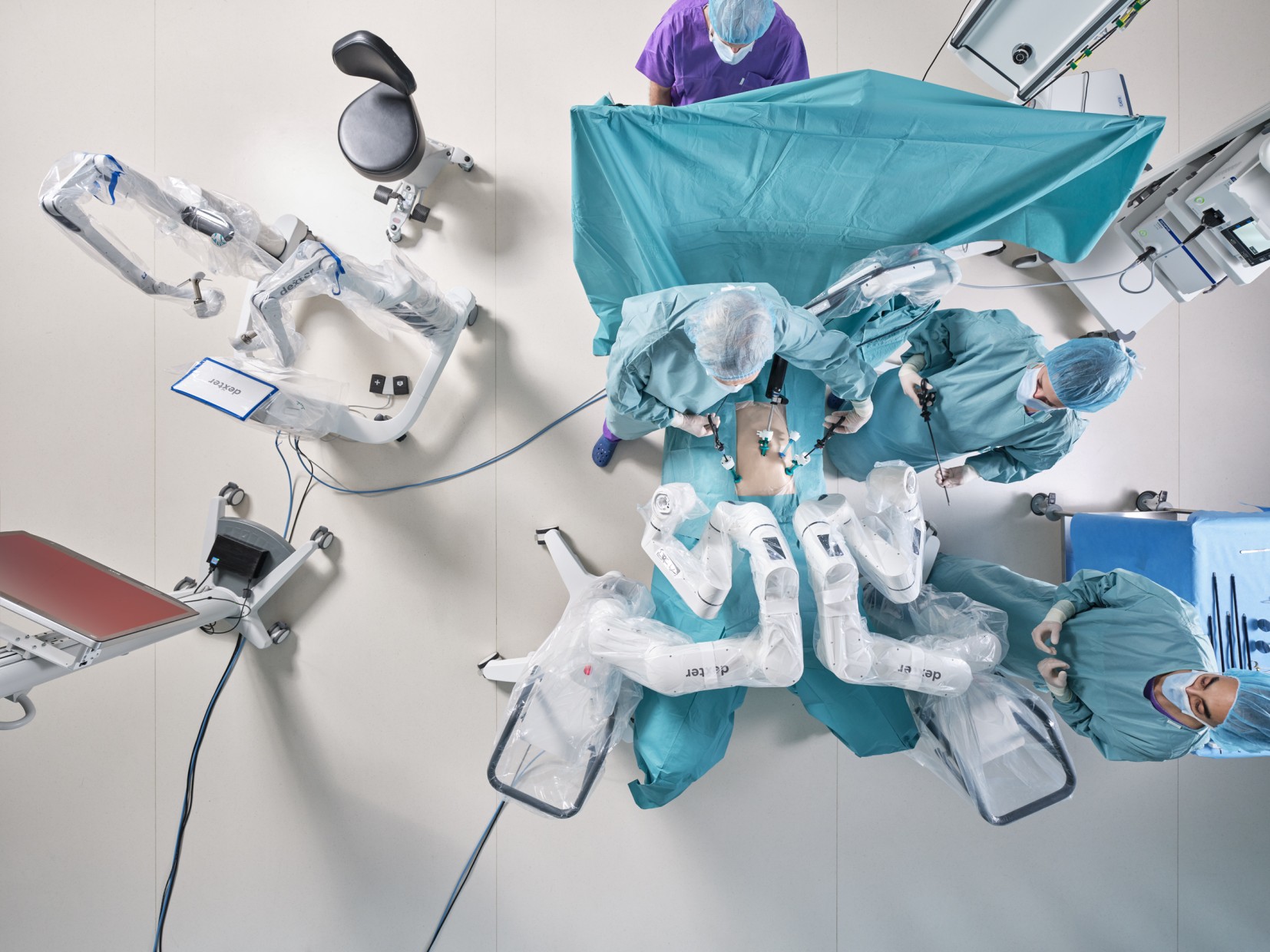
Distalmotion, the global MedTech company empowering access to the benefits of robotic surgery in more sites of care, announced that it has received FDA 510(k) clearance for the use of its DEXTER® Robotic Surgery System in adult cholecystectomy (gallbladder removal). This milestone represents the second approved indication in the US, following De Novo clearance for inguinal hernia repair in Q4 2024.
“Cholecystectomy is a key procedure in general surgery, with approximately one million cases performed annually—of which 60 percent are performed in outpatient settings,” said Greg Roche, CEO of Distalmotion. “With indications for inguinal hernia repair and now cholecystectomy, we’re meaningfully building the reach of DEXTER in the US so that more surgical teams and patients will benefit from robotic surgery. Our mission remains focused on empowering access to robotics with the right robot for the right site of care.”
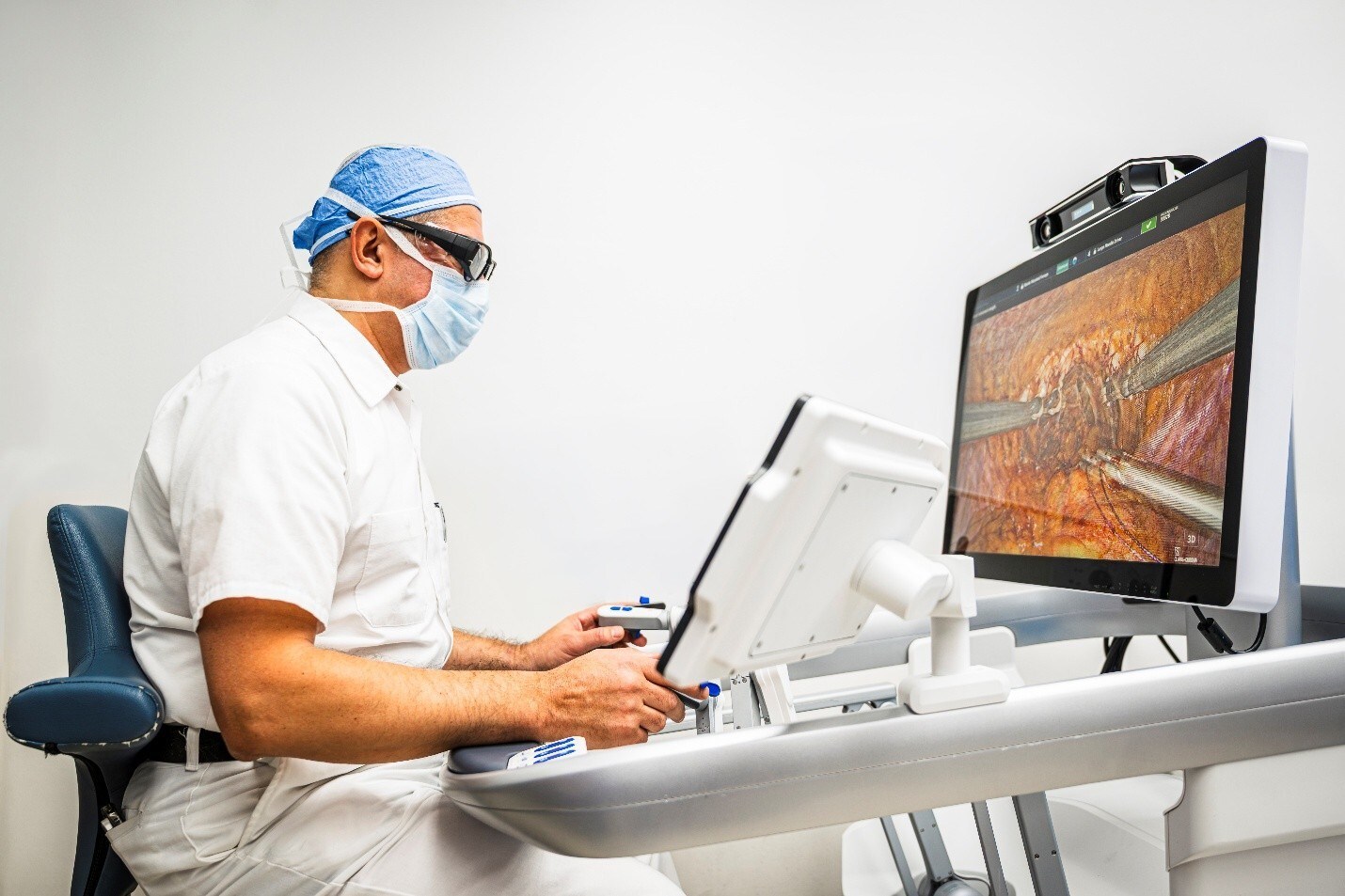
As one of the most frequently performed general surgeries in the United States, cholecystectomy is predominantly carried out in hospital outpatient departments and ambulatory surgery centers (ASCs). As more soft tissue procedures migrate to these settings, there is growing demand for robotic solutions that deliver enhanced dexterity, precision, and surgeon ergonomics—without compromising on flexibility, efficiency, or cost. Purpose-built for outpatient settings, DEXTER features a small, mobile footprint and integrates seamlessly into existing workflows to simplify operations and support high-volume procedures.
Building on its growing footprint in the US and Europe, DEXTER has been used in 2,000 procedures across more than thirty procedure types in general, gynecological, colorectal, and urological surgery. Distalmotion continues to expand access to robotic surgery in the US, with the completion of its pivotal HYPER study (NCT06473675) for benign hysterectomy and ongoing patient enrollment in its sacrocolpopexy clinical trial.