Asensus Surgical Secures FDA 510(k) Clearance for Expanded Robotic Surgery Indication
Senhance Surgical System Approved for Urology Procedures in U.S., Paving the Way for Broader Applications
Asensus Surgical (NYSE:ASXC) announced today that it received FDA 510(k) clearance for an expanded surgical robot indication.
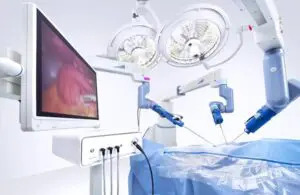
The Research Triangle Park, North Carolina-based company’s Senhance surgical system can now treat adult and pediatric urology patients.
Asensus originally designed Senhance for use in general laparoscopic and laparoscopic gynecological procedures. The company won FDA clearance for the system in 2017. Since then, it secured expanded indications, deals with Google and Nvidia, and hospital placements around the world.
Senhance has been successfully utilized for urology procedures outside the U.S. for years but can now be used for the U.S. patient population.
This marks the latest step forward for Asensus, which also has the next-generation Luna platform under development. The company suggested last year that it targeted 2025 for FDA clearance for Luna. At the start of this year, the company showed Luna off to surgeons, conducting an in vivo lab evaluation of the next-generation surgical robot. The company inked a manufacturing deal for the platform in November as well.
Asensus is also in the process of being acquired by Karl Storz after announcing a deal last month.
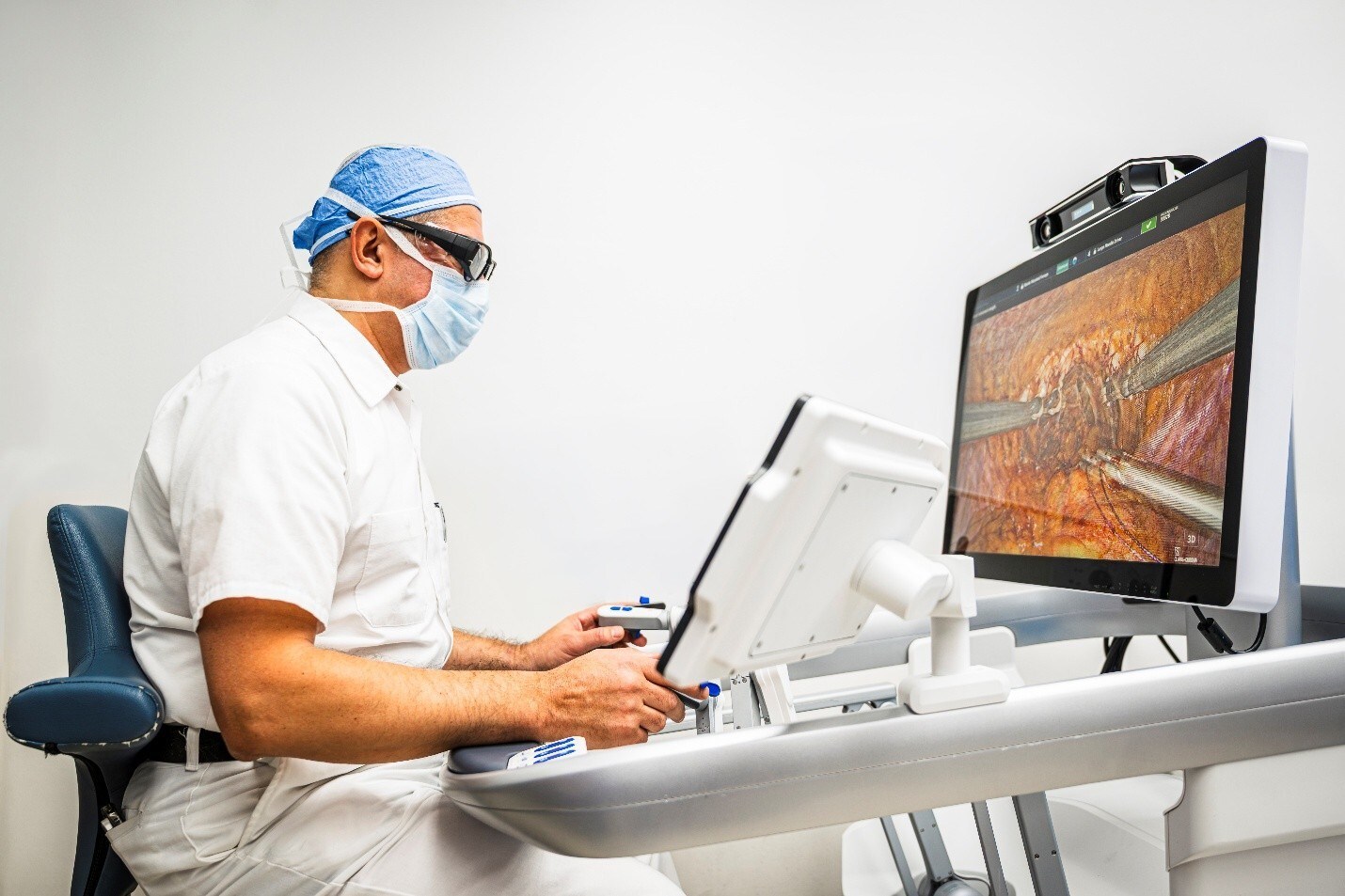
“This FDA clearance marks another milestone for Asensus Surgical and represents an additional indication expansion in the U.S. market,” said Anthony Fernando, Asensus Surgical president and CEO. “The Senhance System’s precision and advanced digital capabilities make it uniquely suited for urological procedures, offering surgeons the benefit of digital tools and smaller instrumentation. We are excited to bring this technology to urologists and their patients across the United States.”