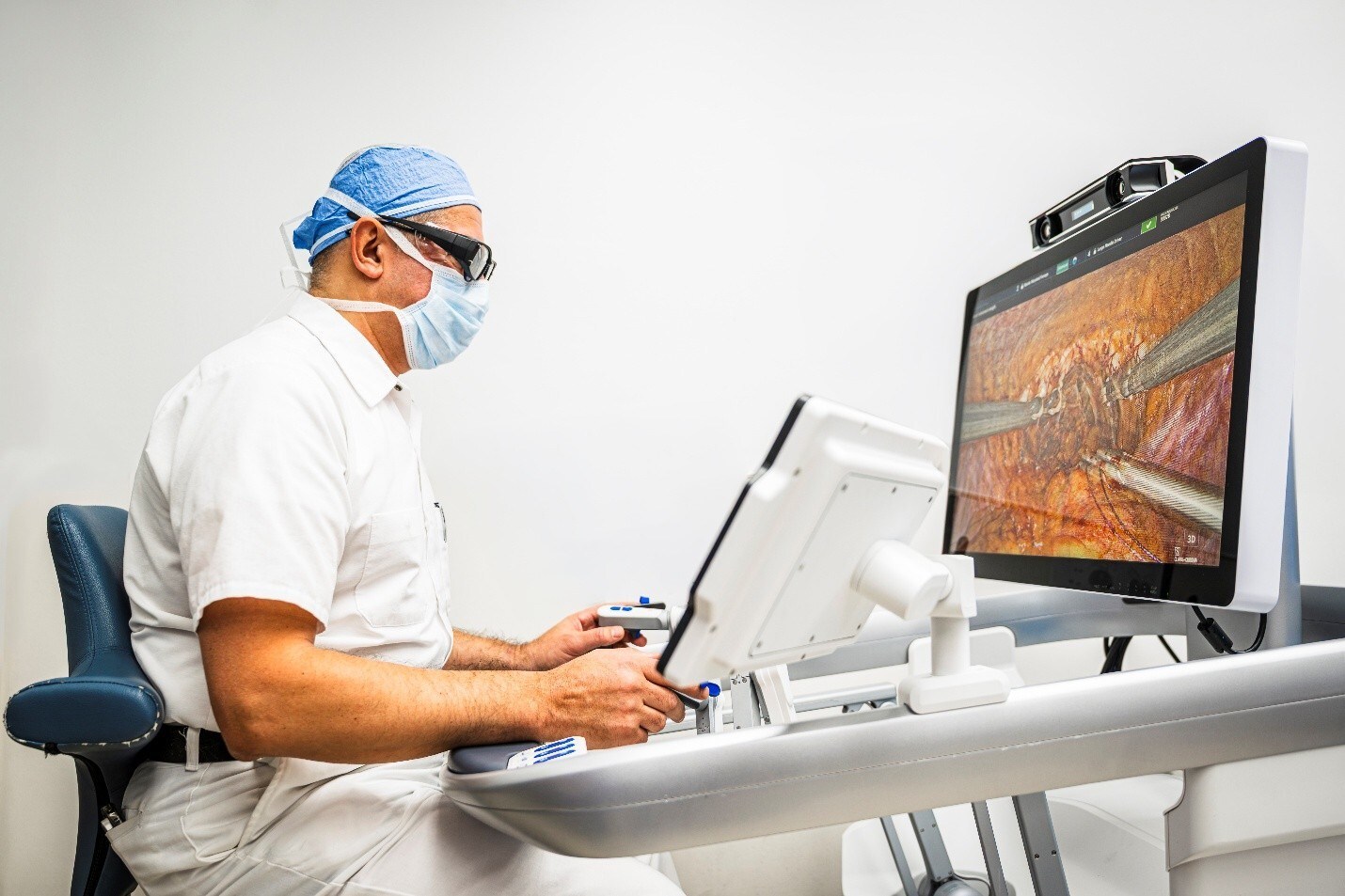
Stereotaxis Receives FDA 510(k) Clearance for Next-Generation GenesisX Robotic System
GenesisX enhances accessibility and workflow efficiency for minimally invasive endovascular surgery with compact design and simplified installation.
Image Courtesy: Public Domain
Stereotaxis, a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, announced that it received U.S. Food and Drug Administration 510(k) clearance for its latest generation robotic system, GenesisX™.
GenesisX is the latest advance in endovascular surgical robotics, building upon the established benefits of Robotic Magnetic Navigation while significantly enhancing the accessibility of the technology for healthcare providers. The system features a compact and efficient design, incorporating magnetic shielding into its structure in place of the shielding otherwise installed in the walls of the operating room. It operates on standard 120/230V power, requires no structural anchoring, and features an 80% smaller system cabinet. These allow for GenesisX to be installed in existing non-modified cath labs without complex architectural planning or construction. The system’s smaller and lighter design enhances workflow efficiency while maintaining the highest standards in speed and responsiveness.
“This is a landmark approval as we transform the accessibility and scalability of Robotic Magnetic Navigation, pioneering broad robotic adoption across endovascular surgery,” said David Fischel, Chairman and CEO of Stereotaxis. “We thank and congratulate all those who helped us reach this milestone. Successful development, approval, and deployment of complex surgical robots that operate reliably in daily clinical use is a unique expertise. This is our second robotic system launched within five years, reflecting our commitment and capacity to drive significant innovation in electrophysiology and endovascular medicine.”
“For years, challenging infrastructure demands limited the adoption of robotic technology amongst the community of physicians interested in its clinical benefits,” said Dr. Francis Marchlinski, Professor of Medicine and Director of Electrophysiology at the University of Pennsylvania Health System. “The GenesisX design changes that by dramatically simplifying installation. This helps to more readily bring the precision of robotic navigation and its ease of use for the operator to a broader patient population.”
Stereotaxis has initiated a limited launch of GenesisX in the United States and Europe while simultaneously expanding its portfolio of compatible catheters, enhancing compatibility with various x-rays, demonstrating commercial use, and enhancing supply chain, manufacturing, installation and commercial processes for a full launch. GenesisX’s flexibility as a capital system provides opportunities for alternative financing models including sales, leases and pay-per-use.