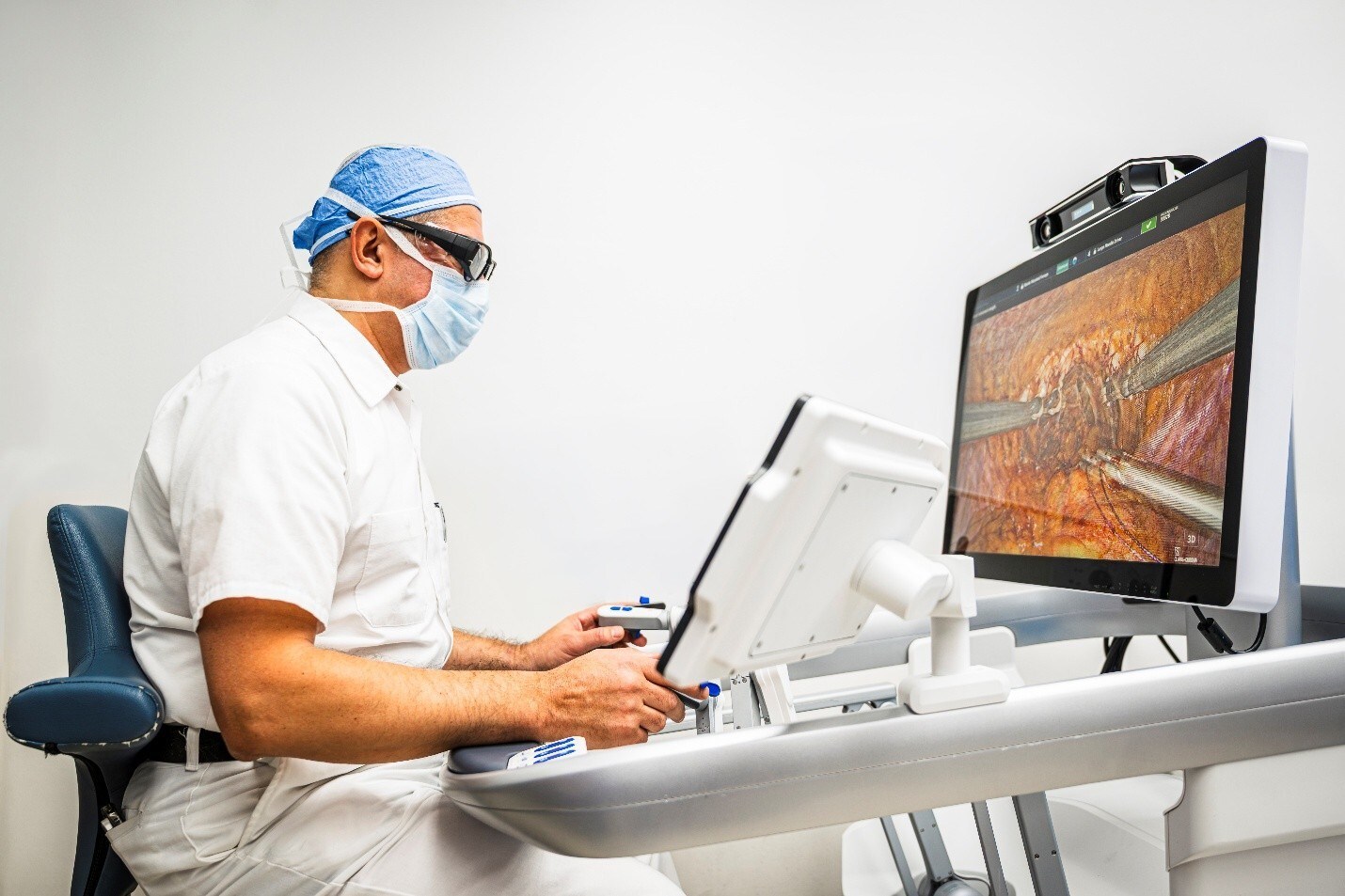
NanoVibronix Launches ENvue Drive™: Robotic Breakthrough for Bedside Navigation
Combining intelligent robotics with FDA-cleared electromagnetic guidance, ENvue Drive™ aims to transform enteral and vascular access by automating bedside procedures—enhancing precision, safety, and clinician efficiency amid growing global demand.
Image Courtesy: Public Domain
NanoVibronix, Inc. (NASDAQ: NAOV) a medical technology company specializing in non-invasive therapeutic systems, today announced the official launch of a next-generation robotic development initiative within its ENvue Medical (“ENvue” or “ENvue Medical”) division. The new platform, ENvue Drive™, is being developed to automate electromagnetic navigation for enteral and vascular access procedures at the bedside.
This significant step reinforces ENvue’s leadership in clinical navigation and addresses the critical unmet need to increase procedural volume with fewer hands at the bedside. The system, now in engineering development, is being designed to integrate with the Company’s FDA-cleared electromagnetic guidance technology for enteral access, offering automated alignment and positional stability, while allowing clinicians to remain in full control.
“ENvue’s Drive platform marks a transformative move for bedside clinical care,” said Dr. Doron Besser, CEO of NanoVibronix and ENvue Medical. “By embedding intelligent robotics into high frequency clinical workflows, starting with feeding tubes and expanding into vascular areas, we believe that we are addressing a major clinical challenge of doing more with fewer hands without compromising precision or safety while giving clinicians a new level of confidence and consistency in care.
“Importantly, the Drive platform is a scalable foundation for intelligent bedside navigation. Through this initiative, we believe we are building a future-ready solution portfolio that can evolve with clinical demands across multiple care settings and drive growth.”
Addressing a Growing Global Demand
Each year, tens of millions of nasoenteric tube and vascular access procedures are performed globally. ENvue’s new robotic platform is positioned to address a growing clinical need to automate high-volume, bedside procedures in the face of workforce shortages and increasing patient complexity.
Key Market Drivers :
- The global enteral feeding devices market is projected to reach $3.99 billion by 2030, driven by aging populations, higher procedural complexity and the rise in chronic illness (Source: Grand View Research, 2024).
- The vascular access device market, including PICC lines, is currently valued at $6.2 billion, forecasted to exceed $10.5 billion by 2030 (Source: Markets and Markets, 2024).
- In the United States alone, over 10 million nasoenteric feeding tubes are placed annually (Source: internal market data purchased by ENvue Medical, 2025).
- The robotic catheter navigation segment is expected to grow at over 14% CAGR, reaching $500 million by 2030 (Source: BIS Research, 2023).
Dr. Besser continued, “Despite these massive procedural volumes, to our knowledge, no intelligent robotic system currently exists to assist with electromagnetic navigation during enteral or vascular access at the bedside. ENvue Drive aims to fill this gap.”
Designed to Assist, Not Replace
ENvue Drive is being developed to augment clinician performance, not replace it, particularly in critical care settings such as ICUs, step-down units and telemetry wards.
Development goals include:
- Automated bedside alignment of ENvue’s electromagnetic probe
- Real-time, stabilized positioning during nasoenteric and PICC procedures
- Reduced need for multiple clinicians to perform a procedure, ideal during nurse shortages
- Hands-free functionality for increased safety, consistency and reproducibility
- Seamless integration with existing ENvue consoles and software
Development and Regulatory Status
The robotic platform is currently in preclinical development phase, with engineering prototypes in process and clinical advisor input underway. The system has not yet been submitted to the U.S. Food and Drug Administration (FDA) and is not yet cleared for clinical use.
The Company expects to initiate formal regulatory planning and submission milestones later this year and anticipates having a functional prototype by year-end. All future development remains subject to applicable regulatory approvals.
Building on Recent Strategic Momentum
This announcement follows a strong second quarter for NanoVibronix and ENvue Medical including:
- Execution of a dual-system acquisition agreement with a major Northeastern U.S. hospital, reinforcing ENvue’s momentum in adult enteral feeding tube navigation.
- Issuance of a new U.S. patent protecting ENvue’s electromagnetic guidance system for pediatric feeding applications.
- Continued geographic expansion across the Southeast, Midwest, and Northeastern U.S., with growing market penetration.
Dr. Besser, concluded, “In the quarters ahead, we expect to share meaningful updates on our hardware and software development progress, new clinical collaborations and advisory board appointments, as well as our regulatory roadmap. We are also actively pursuing technical partnerships, AI-powered capabilities and clinical research initiatives to further strengthen the impact and scalability of our platform.”