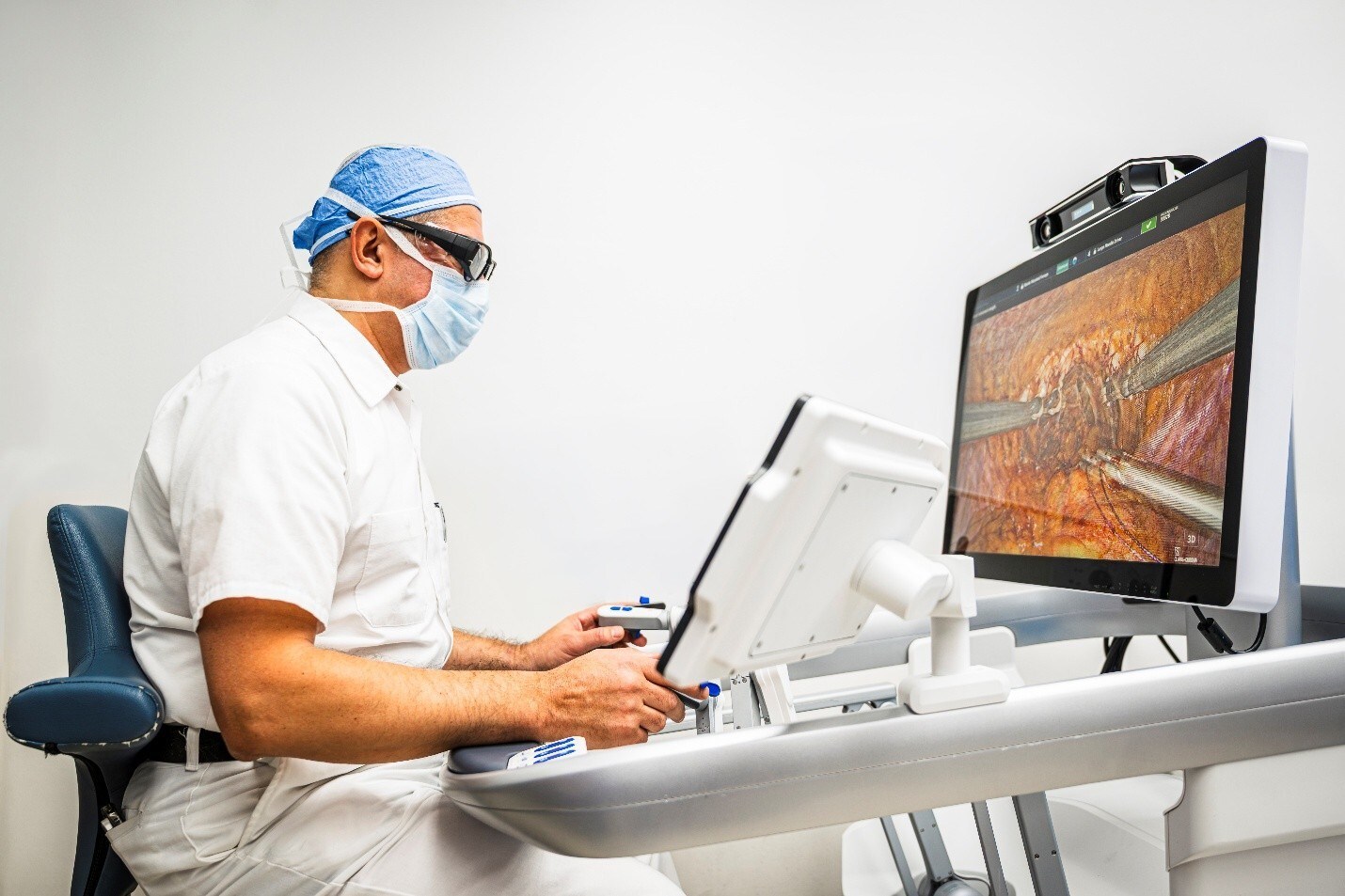
Stereotaxis Secures First Order for GenesisX™, Its Next-Generation Robotic Surgery System
Pioneering European hospital to establish the first GenesisX robotic lab, marking a key milestone toward full commercial launch and broader adoption of robotic endovascular surgery.
Image Source | Public Domain
Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, announced it has received the first order for its latest generation robotic system, GenesisX™.
“We are thrilled to announce the first firm order for GenesisX from a pioneering European hospital,” said David Fischel, Stereotaxis Chairman and CEO. “Establishing the first GenesisX robotic lab is a critical milestone for us as we advance towards full commercial launch. We look forward to demonstrating the performance and reliability of GenesisX in the demanding clinical environment. GenesisX promises to transform the accessibility of Robotic Magnetic Navigation and is central to our mission of driving broad robotic adoption throughout endovascular surgery.”
GenesisX is the latest advance in endovascular surgical robotics, building upon the established benefits of Robotic Magnetic Navigation while significantly enhancing the accessibility of the technology for healthcare providers. GenesisX utilizes smaller magnets and incorporates magnetic shielding into its structure in place of the shielding otherwise installed in the walls of the operating room. It requires no structural anchoring through the floor and operates using standard 120/230V power outlets. A single fiber is routed from each robot to the system cabinet, which is 80% smaller than previous cabinets and can fit under a table in the operating room. GenesisX is smaller and lighter than any previous generation system, while maintaining the highest standards in speed, responsiveness, and efficient workflow.
GenesisX obtained CE Mark in Europe in 2024 and has been submitted to the FDA for 510(k) clearance in the United States. During 2025, Stereotaxis plans to gain regulatory approvals in Europe and the US for a portfolio of compatible EP and vascular catheters, demonstrate real-world use of GenesisX, enhance compatibility of GenesisX with various x-rays, and prepare supply chain, manufacturing, installation and commercial processes for a full launch.