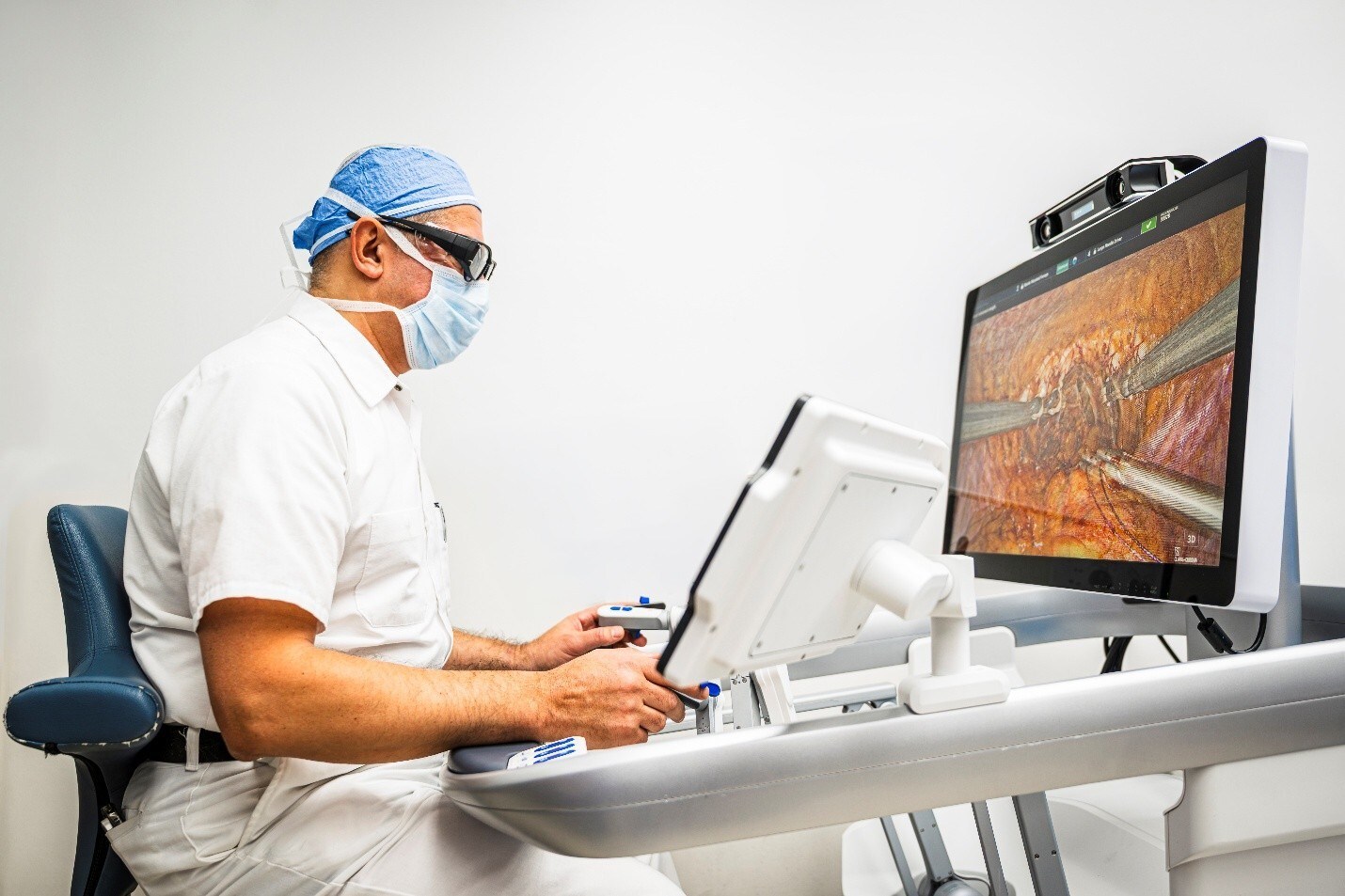
Microbot Medical Launches Commercial Availability of LIBERTY® Robotic System in the U.S.
Following FDA clearance, the world’s first single-use, remotely operated robotic system for endovascular procedures enters Limited Market Release ahead of full launch in 2026.
Image Courtesy: Public Domain
Microbot Medical Inc. (Nasdaq: MBOT), developer and distributor of the innovative LIBERTY® Endovascular Robotic System, announced that its LIBERTY® System, the first FDA cleared single-use, remotely operated robotic system for peripheral endovascular procedures, is now commercially available in the U.S. The Limited Market Release (LMR) will introduce LIBERTY® to selected high procedure volume regions where the Company already experienced preliminary demand for LIBERTY®. The LMR will focus on collecting real-world insights from potential high-volume users to guide responsible growth and ensure consistent quality and performance, leading to the expected Full Market Release (FMR) during the Society of Interventional Radiology (SIR), the largest U.S. medical conference for Interventional Radiology, in April 2026.
“We are excited to enter the commercialization phase of LIBERTY®. We are building a new robotic category with the introduction of LIBERTY®, the world’s first single-use robotic system. Launching just weeks after announcing FDA clearance, we believe that we have demonstrated the strength of our team and the unique innovation behind LIBERTY®,” commented Harel Gadot, CEO, President and Chairman. “Building on the strong interest and feedback we have received so far from physicians and hospital administrators, the limited market release is expected to enable us to responsibly support early adopters and lay the groundwork for a full market launch at the Society of Interventional Radiology meeting in April.”
Since receiving the FDA 510(k) clearance for the LIBERTY® System in September, the Company has advanced its commercial readiness by securing a third-party logistics partner and expanding its commercial leadership team to ensure a fully supported and successful limited market release.